Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
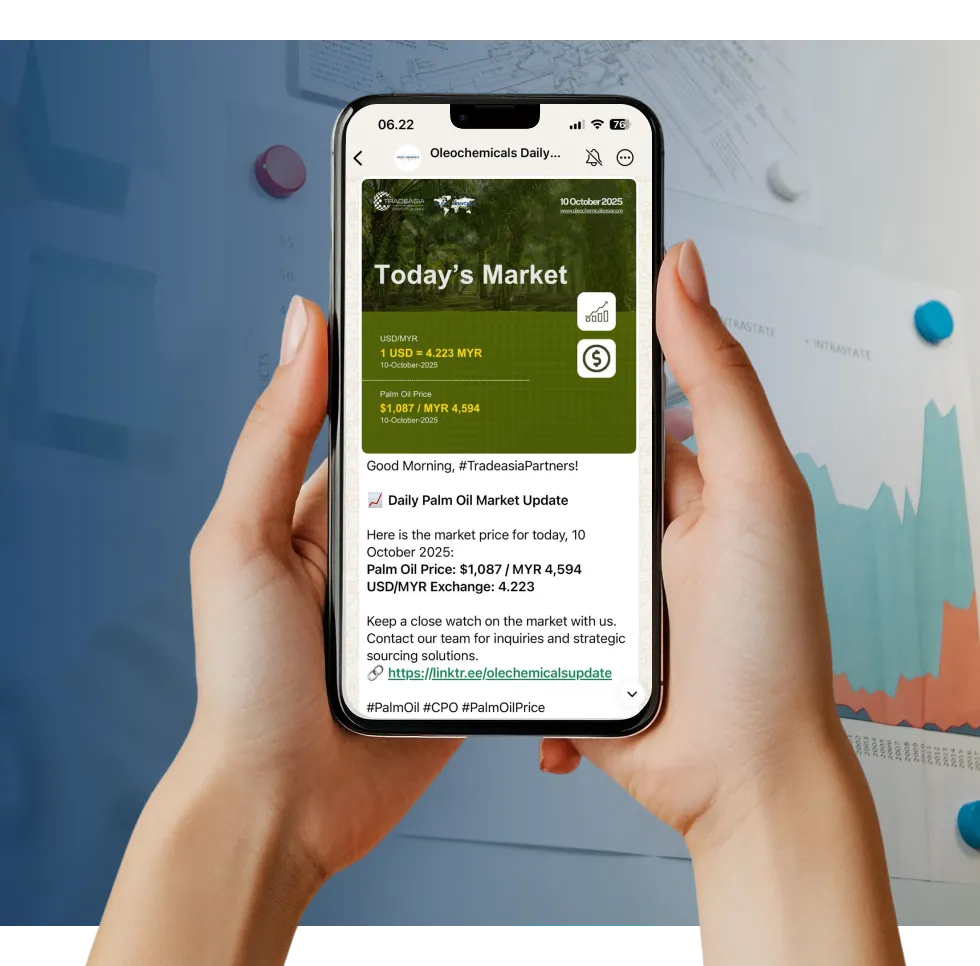
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates
Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Log in now to access technical product documents from our product range.
Don't have an account? Sign Up Here
Home All Products Sodium Acetate
|
IUPAC Name |
: Sodium Ethanoate |
|
Cas Number |
: 127-09-3 |
|
HS Code |
: 2915.22.00 |
|
Formula |
: CH3COONa |
|
Appearance Name |
: White Crystalline Powder |
|
Common Names |
: Sodium Acetate Anhydrous |
|
Packaging |
: 25 Kg Plastic Woven Bags |
For more detailed information including pricing, customization, and shipping:
Brief overview
Sodium acetate is the sodium salt of acetic acid with chemical formula is C2H3O2Na. Sodium acetate is an ionic compound with all the common physical properties of ionic substances. Sodium acetate may be found as an anhydrous salt or a trihydrate. Sodium acetate is a cheap chemical with a wide range of uses.
Manufacturing Process
Sodium acetate can be produced industrially by reacting large amounts of glacial acetic acid with sodium hydroxide, as seen in the following reaction.
C2H4O2 + NaOH → NaO2CCH3 + H2O
Food Industry
Sodium acetate is used as a food additive and added to food to help prevent bacterial growth. Sodium acetate can be used as a neutralizer for basic or alkaline foods and as a buffer to help maintain a specific pH. Sodium acetate can also enhance flavor, and there are no known adverse effects on human health. Sodium acetate is used as a seasoning in the food industry for salt and vinegar flavor.
Chemical Industry
Sodium acetate neutralizes the sulphuric acid found in waste streams from textile industries. It is also used as a photoresist when aniline dyes are used. In the rubber industry, it delays the vulcanization of chloroprene in synthetic rubber. It acts as a sealant in concrete and lessens water permeation to prevent damage. It also helps remove stains, rust, and other impurities from metallic surfaces.
Heating Pad
A flat, uneven metal disc is placed in the solution. A small amount of sodium acetate crystals is released when the disk moves. These crystals start a chain reaction of crystallization with the rest of the sodium acetate. This reaction is instantaneous, releasing a lot of energy stored in the sodium acetate crystal structure. The crystallization of sodium acetate is exothermic. This principle is used in heating pads. The heating pad is reusable since sodium acetate can return to a supersaturated liquid state when placed in boiling water and cooled to room temperature.

