Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
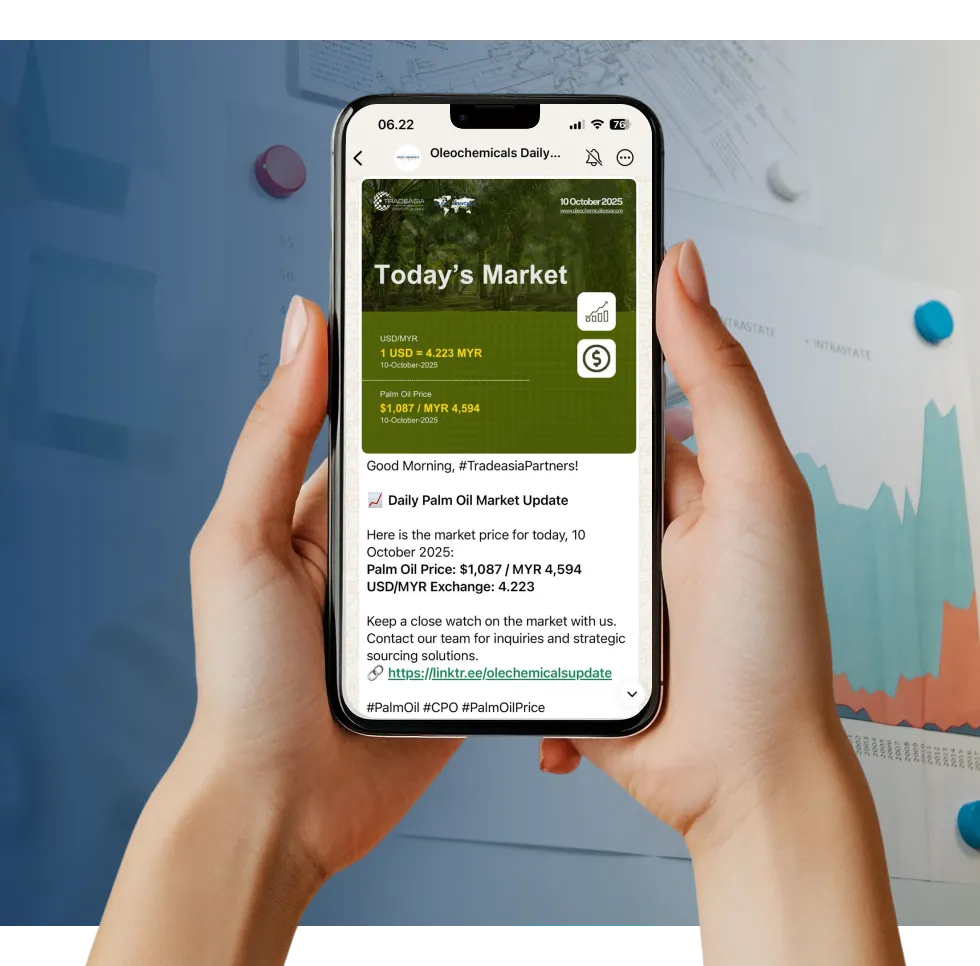
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates
Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Log in now to access technical product documents from our product range.
Don't have an account? Sign Up Here
Home All Products Phthalic Anhydride (99,8%) - India
|
IUPAC Name |
: 2-Benzofuran-1,3-dione |
|
Cas Number |
: 85-44-9 |
|
HS Code |
: 2917.35.00 |
|
Formula |
: C8H4O3 |
|
Appearance Name |
: Fine White Powder |
|
Common Names |
: Isobenzofuran-1,3-dione, Phthalic anhydride |
|
Packaging |
: 25 KG Plastic Bag, 600 KG Big Plastic Bag |
For more detailed information including pricing, customization, and shipping:
Brief Overview
Phthalic anhydride, represented by the formula C6H4(CO)2O, serves as the anhydride of phthalic acid and is a significant commercial form of the acid. It marked the pioneering use of a dicarboxylic acid anhydride on a large scale, with this white solid playing a crucial role in industrial chemistry, particularly in the extensive production of plasticizers for plastics. The estimated global production volume in 2000 reached about 3 million tonnes annually.
Manufacturing Process:
Discovered by Auguste Laurent in 1836, phthalic anhydride is currently synthesized through various methods, including the oxidation of naphthalene or ortho-xylene. In these processes, vanadium pentoxide (V2O5) serves as the active oxidant, playing a key role in multiple steps and being regenerated by molecular oxygen. Starting from o-xylene, the oxidation reaction is run at about 320–400 °C and has the following stoichiometry:
C6H4(CH3)2 + 3 O2 → C6H4(CO)2O + 3 H2O
The reaction proceeds with about 70% selectivity. About 10% of maleic anhydride is also produced: C6H4(CH3)2 + 7.5 O2 → C4H2O3 +4 H2O + 4 CO2
Phthalic anhydride and maleic anhydride are recovered by distillation by a series of switch condensers.
The naphthalene route (the Gibbs phthalic anhydride process or the Gibbs–Wohg naphthalene oxidation reaction), a process whose use has declined in compared to the o-xylene route, has the following mechanism:

Phthalic anhydride can also be prepared from phthalic acid by simple dehydration.
Phthalate esters plasticizers:
Phthalic anhydride's primary application lies in being a precursor to phthalate esters, crucial plasticizers for vinyl chloride. These esters, produced through the alcoholysis reaction with phthalic anhydride, reached an annual production of approximately 6.5×109 kg in the 1980s, showcasing an increasing scale each year, all originating from phthalic anhydride. Additionally, it finds significant use in manufacturing polyester resins, along with minor applications in alkyd resins for paints and lacquers, certain gums, insect repellents, and urethane polyester polyols.
Precursor to dyestuffs:
In the realm of dyestuff production, phthalic anhydride is extensively utilized, notably in the synthesis of anthroquinone dye quinizarin through reactions with para-chlorophenol followed by chloride hydrolysis. Phenolphthalein, a well-known compound, results from the condensation of phthalic anhydride with two equivalents of phenol under acidic conditions, hence its name.
Pharmaceuticals:
In the pharmaceutical sector, phthalic anhydride, when treated with cellulose acetate, yields cellulose acetate phthalate (CAP), a common excipient for enteric coatings. CAP has demonstrated antiviral activity, and phthalic anhydride itself is a degradation product of CAP.

